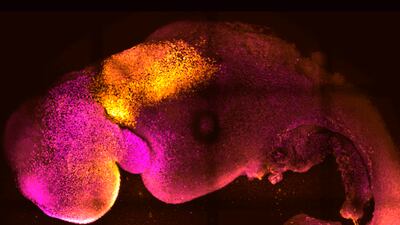
Researchers at the University of Cambridge have developed “synthetic” model embryos with brains and beating hearts without the use of eggs or sperm.
The team instead used stem cells — the body’s master cells that can become almost any type of cell in the body — taken from mice, to create the embryos.
The development, led by Prof Magdalena Zernicka-Goetz over 10 years, could help researchers understand why some embryos fail while others go on to develop into a healthy pregnancy.
Additionally, experts say the results could be used to guide repair and development of synthetic, or artificial, human organs for transplantation.
Prof Zernicka-Goetz, who specialises in mammalian development and stem cell biology at Cambridge’s Department of Physiology, Development and neuroscience, said: “Our mouse embryo model not only develops a brain, but also a beating heart, all the components that go on to make up the body.
“It’s just unbelievable that we’ve got this far.
“This has been the dream of our community for years, and a major focus of our work for a decade and finally we’ve done it.”
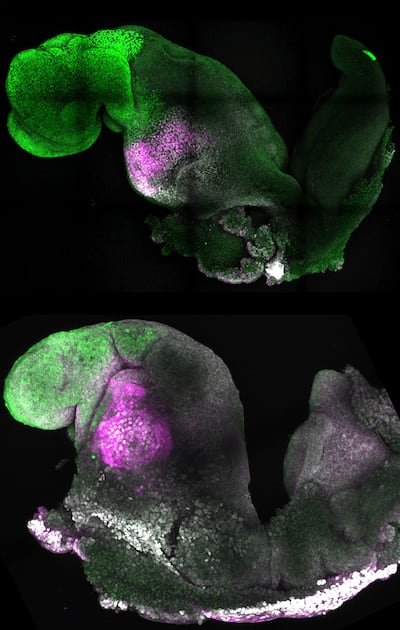
Although the current research was carried out in mouse models, the researchers are developing similar human models which could help understand mechanisms behind crucial processes that would be otherwise impossible to study in human embryos.
UK law currently permits human embryos to be studied in the laboratory only up to the 14th day of development.
If the methods developed are shown to be successful with human stem cells in future, they could also be used to guide development of synthetic organs for patients awaiting transplants.
Prof Zernicka-Goetz said the work could also lead to the development of new ways to heal adult organs “using the knowledge we have on how they are made”.
In order for a human embryo to successfully develop, there needs to be a dialogue between the tissues that will become the embryo, and the tissues that will connect the embryo to the mother.
In the first week following fertilisation — the point at which human life begins — three types of stem cells develop.
One of these will eventually become the tissues of the body, another will become the placenta, and the other is the yolk sac, where the embryo grows and where it gets its nutrients from in early development.
Many pregnancies fail at the point when the three types of stem cells begin to send mechanical and chemical signals to each other, which tell the embryo how to develop properly.
The team of researchers have focused on trying to ascertain why some pregnancies fail and some succeed.
“The stem cell embryo model is important because it gives us accessibility to the developing structure at a stage that is normally hidden from us due to the implantation of the tiny embryo into the mother’s womb,” Prof Zernicka-Goetz said.
“This accessibility allows us to manipulate genes to understand their developmental roles in a model experimental system.”
The team put together cultured stem cells representing each of the three types of tissue in the right proportions and environment to promote their growth and communication with each other. Eventually, all three components self-assembled into an embryo.
“This period of human life is so mysterious, so to be able to see how it happens in a dish — to have access to these individual stem cells, to understand why so many pregnancies fail and how we might be able to prevent that from happening — is quite special,” Prof Zernicka-Goetz added.
Researchers say a big advance in the study is the ability to generate the entire brain which has been a major goal in the development of synthetic embryos.
The findings are published in the journal Nature.