The UK medicines regulator approved the Covid-19 vaccine made by Pfizer for children aged 12 to 15.
The Medicines and Healthcare Products Regulatory Agency concluded that the drug was safe and effective for use in younger people, with the benefits outweighing any risk.
“We have in place a comprehensive safety surveillance strategy for monitoring the safety of all UK-approved Covid-19 vaccines and this surveillance will include the 12-to-15-year age group," MHRA chief executive Dr June Raine said.
“No extension to an authorisation would be approved unless the expected standards of safety, quality and effectiveness have been met."
The drug is already approved for use in the UK for those over 16.
The UAE, EU and US in recent weeks gave the green light for Pfizer's vaccine to be given to children aged 12 to 15. Some European countries have already started inoculating teenagers with the drug.
While young people are less likely to succumb to serious illness or death from Covid-19, they can still spread the disease to more vulnerable people. Real-world studies in England showed that a single dose of a Covid-19 vaccine can reduce transmission by up to half.
The MHRA said Britain's Joint Committee on Vaccination and Immunisation would decide whether to extend the country's vaccination programme to those aged 12 and above.
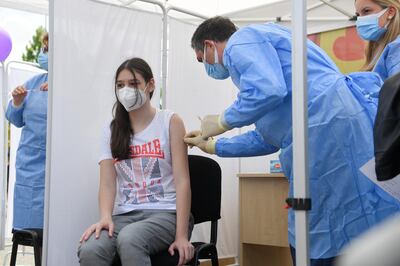
Prof Munir Pirmohamed, chairman of the Commission on Human Medicines, who is advising the government, said Friday's decision was taken after a trial involving 2,000 children aged 12 to 15.
"There were no cases of Covid-19 from seven days after the second dose in the vaccinated group, compared with 16 cases in the placebo group," he said.
"In addition, data on neutralising antibodies showed the vaccine working at the same level as seen in adults aged 16 to 25 years. These are extremely positive results.”








