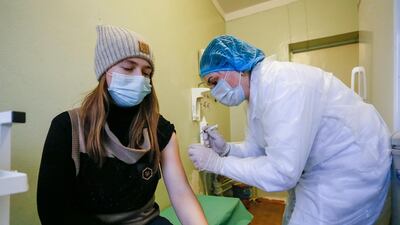
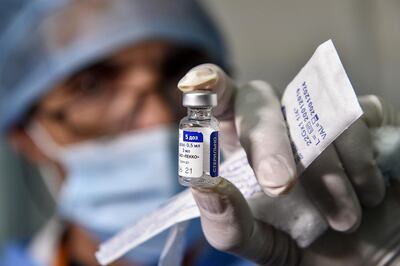
Russia's Sputnik V vaccine is highly effective against symptomatic Covid-19, a trial carried out among almost 15,000 participants in Moscow has found.
Data from clinical trials, published in The Lancet medical journal on Tuesday, showed the vaccine was 91.6 per cent effective.
The positive results were published on the same day the first shipment of the AstraZeneca/Oxford University vaccine arrived in Dubai after it was approved for use.
The first shipment contained 200,000 doses, which is enough for 100,000 people.
The Sputnik V vaccine, which uses similar technology to the Oxford drug, was well-tolerated and worked in the elderly as well, the peer-reviewed study showed.
"From 21 days after receiving the first dose (the day of dose 2), 16 cases of symptomatic Covid-19 were confirmed in the vaccine group and 62 cases in the placebo group – equivalent to an efficacy of 91.6 [per cent]," a report summary said.
British researchers said the results showed the "scientific principle of vaccination is demonstrated", and quelled western doubts that its development was rushed.
Sputnik V has been approved for use in 16 countries, including the UAE. Hungary became the latest to approve the vaccine, acting independently of the EU medicines regulator, which is yet to authorise the drug.
Ian Jones, professor of virology at the University of Reading, told The National that he would take the vaccine based on the data. He urgedwestern medical regulators to consider approving it in their countries.
"I don’t see any reason not to," he said. "[Russia] deserves credit where credit is due."
The report’s authors said 219,77 adults were assigned either the vaccine or a placebo at hospitals and clinics in Moscow. Sixteen cases of symptomatic Covid-19 were identified in the vaccine group at least 21 days after the first dose, compared with 62 cases in the placebo group.
Sixty-eight participants became ill during the trial and four died but the authors said the deaths were not associated with the vaccine. Those who fell ill experienced a mild, flu-like sickness or weakness, the authors said.
It was acknowledged that more research was needed on the vaccine’s effectiveness among different racial groups as most participants in the trial were white, while the study only detected Covid-19 cases in people who reported symptoms.
"Further research is needed to understand the efficacy of the vaccine on asymptomatic Covid-19," The Lancet said.
However, Prof Jones said people should not be unduly concerned by the fact only symptomatic participants were tested.
"In my view the current almost obsession that you must prevent the virus from circulating is not justified," he said. "The goal should be that you want to stop people from dying and this study shows that the vaccine was effective in preventing severe illness from Covid.
"Covid may well join the batch of respiratory viruses that crop up every year, so surely the emphasis must be on preventing severe illness."
Sputnik V is an adenoviral vector vaccine using similar technology to the Oxford/AstraZeneca dose. The vaccine needs to be stored at minus 2.8°C, making it easier to distribute than those requiring ultra-cold storage.
The peer-reviewed publication may help Russian efforts to vaccinate 60 per cent of its adult population in the first half of the year after a slow start. The homegrown shot has faced doubts at home. Only 38 per cent of Russians are ready to take Sputnik V, a poll conducted in December by the Levada Centre showed.
Many vaccination centres in Moscow, where the campaign is largest, are not working at capacity even after President Vladimir Putin ordered universal access to the inoculation in mid-January. The Russian president hasn't yet said when he would get vaccinated.
Sputnik V provides protection against Covid-19 for over a year, and can be employed again for re-vaccination, its developers said.