Just as Russia made a statement by calling its Covid-19 vaccine Sputnik V, so Cuba sent a message by naming some of the coronavirus vaccines it is developing "Soberana" – Spanish for sovereign.
Cuba’s self-reliance is born of necessity.
Relatively poor, especially after a year in which its tourist industry has been battered by the pandemic, and heavily isolated by US sanctions, the country would have struggled to secure supplies of the Pfizer and Moderna coronavirus vaccines in particular.
Rather than join Covax – the global programme to provide vaccines to poor countries – Cuba has used its decades-long expertise in biotechnology to develop and produce its own.
"It's partly or largely the result of a strategic development policy to invest in science and technology for social development," said Dr Helen Yaffe, a lecturer in economic and social history at the University of Glasgow in the UK and author of We Are Cuba!: How a Revolutionary People Have Survived in a Post-Soviet World.
"In the case of Cuba developing its vaccines, it's the necessity – most global south countries have that – combined with the capability."
That capability, in the form of multiple research institutes that co-operate closely with universities and hospitals, has been channelled into the development of vaccines employing tried-and-tested technology.
Initial clinical trials began last year, and Iran has become involved in recent months.

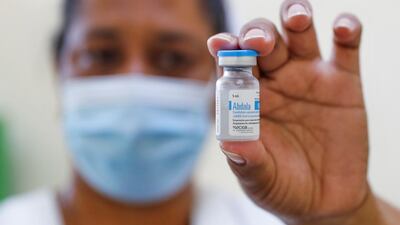
Among Cuba’s vaccines are several named Soberana developed by Havana’s Finlay Institute of Vaccines.
These include Soberana 02, a "conjugate" vaccine consisting of part of the coronavirus spike protein linked or conjugated to a harmless form of the tetanus toxin, which is used to stimulate a stronger immune response.
Soberana 02 has 62 per cent efficacy after two of its three doses, according to Cuban officials.
Official data indicates that another Cuban vaccine, Abdala, made from SARS-CoV-2 proteins and produced by Cuba’s Centre for Genetic Engineering and Biotechnology, had 92.28 per cent efficacy in clinical trials.
Using its own vaccines, Cuba has administered 5.11m doses, and 21 per cent of the population of more than 11m have had at least one jab, according to the University of Oxford’s Ourworldindata website.
"By the end of this year, there’s no doubt the population [of Cuba] will be vaccinated – using the first the vaccine developed in Latin America and the Caribbean," Dr Yaffe said.
“How many countries will be able to say they vaccinated their entire population with their own vaccine?”
Cuba's vaccine programme will have more than domestic significance: the country is likely to export vaccines widely and at low cost, with Venezuela and Ukraine among likely recipients.
"They will charge cost price plus a little bit more to plough into their healthcare system," Dr Yaffe said.
“It’s important politically for Cuba … They won’t make a massive amount of money. But the economic situation is so bad that anything will help.”
Unlike Cuba, Brazil has secured access to multiple foreign Covid-19 vaccines, helped by hosting clinical trials. But is also working on its own, including ButanVac, which officials say could be produced without having to import materials.
It uses a viral vector to stimulate an immune response against coronavirus spike proteins and, crucially, is likely to be inexpensive, making it attractive to Brazil itself and other developing nations.
This month, Brazil’s health regulatory agency, Anvisa, gave the go ahead for clinical trials, and tens of millions of doses could reportedly be available later this year.
While there are existing Covid-19 vaccines with very high efficacy, Prof Eskild Petersen, of the University of Aarhus in Denmark, and chairman of the emerging infections taskforce at the European Society of Clinical Microbiology and Infectious Diseases, says it is good that “competing technologies” are being worked on.
"If you develop a vaccine in Brazil or Cuba and can prove the efficacy and profile of side effects are as good as the best we have – AstraZeneca and Pfizer – then they can probably produce it cheaper," he said.
Among the other Covid-19 vaccines emerging from developing nations is Corbevax from Biological E, a company based in Hyderabad in India.
Developed in partnership with two US institutions, this two-dose vaccine uses components of the coronavirus's spike protein to stimulate an immune response and is said to have performed well in early clinical trials.
Described as costing about half as much as the next-most-expensive jab used in India, it has attracted interest from the Indian government, which this month reserved 300m doses.
Also in June, emergency approval was given for Iran’s domestically developed vaccine, CovIran Barekat, which is made using inactivated coronavirus particles. Iran’s supreme leader, Ayatollah Ali Khamenei, was among the recipients.
Iran, which has also imported Covid-19 vaccines, is set to start late-stage clinical trials of another vaccine, Razi Cov Pars, based on coronavirus spike proteins, in August.
While some developing nations work on their own shots, Covid-19 vaccine supply globally remains highly uneven, with, for example, fewer than one per cent of Africa's population fully inoculated.
Those working in the field recognise that poorer regions need to develop, if not vaccines, then at least manufacturing capacity.
“Vaccine nationalism disappears once we all have the ability to make vaccines,” Dr Adam Ritchie, a senior project manager in vaccine development at the University of Oxford, wrote earlier this year.
“The more we rely on sharing between countries with their own interest, the harder it is to get the vaccine to everyone.”
This was demonstrated recently when India, home to the world’s biggest vaccine maker, the Serum Institute of India, shut down exports in order to maximise domestic vaccination rates amid its second wave.
Prof David Taylor, emeritus professor of pharmaceutical and public health policy at University College London, says "the real challenge is in production", as manufacturing capacity may be more critical than access to the intellectual property of a vaccine.
"My own feeling is that the IP issues have been exaggerated," he said.
“The real challenge is getting enough big-scale capital investment into the production of vaccines wherever you’re doing it.”
Reports indicate that Egypt’s state-supported Vacsera facility is set to soon start production of a Covid-19 shot from China’s Sinovac, making it the second nation in Africa – after South Africa – to manufacture coronavirus vaccines.
As well as catering to local need – less than three per cent of Egypt’s population has had at least one jab – Egyptian-made vaccines are likely to also be exported.
Elsewhere on the continent, the Pasteur Institute of Dakar in Senegal is scheduled to begin packaging and distributing vaccines produced by Belgian’s Univercells by early next year, and will subsequently start actual manufacturing.
It was also recently announced that the World Health Organisation would work with South African companies, universities and the Africa Centres for Disease Control and Prevention to set up an mRNA Covid vaccine technology transfer hub to train manufacturers from poorer countries.
While there are constraints on supply now, Prof Taylor is confident these will ease.
"It don't think there's any doubt we will have enough capability in two or three years' time," he said.
“[But] it’s terribly difficult for the people facing a big challenge to say there’s no adequate vaccine supply now.”