Researchers in Abu Dhabi, who developed a stem cell treatment for Covid-19 that showed promising results, have taken a step towards making sure the method can be used widely to benefit more patients.
The technique, developed by a team of doctors and researchers at Abu Dhabi Stem Cell Centre, was announced last month after it was found to be effective when supplementing standard medical care.
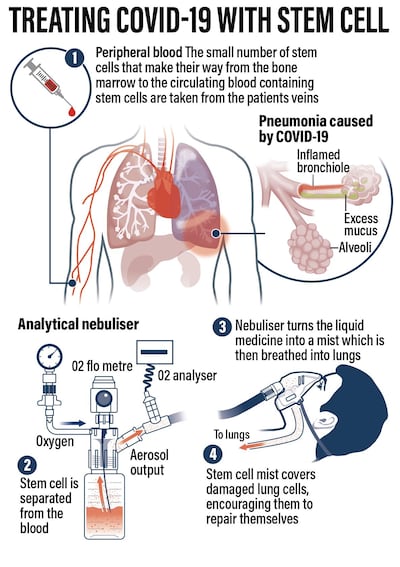
The treatment involves extracting stem cells from the patient’s own blood and reintroducing them as a nebulising mist after reactivating them.
Initial results from the treatment, administered in the UAE to 73 Covid-19 patients with moderate to severe symptoms, were promising, with all patients responding well to the treatment. But researchers stressed at the time that the results were preliminary and further analysis of the data was needed.
That analysis has now shown the treatment to be effective and safe as an add-on to other forms of medical care.
On Monday, it was announced that the researchers secured intellectual property rights protection for the technique, opening the way for it to be widely shared.
"The patients that received stem cells therapy improved faster than those who received the standard treatment only," said Dr Fatema Alkaabi, specialist haematologist at Sheikh Khalifa Medical City.
"Patients who received the stem cells treatment demonstrated clinical improvement within the first four days of treatment as evidenced by lower severity scores. The standard treatment group took eight days to show similar findings."
She said severe patients who received the treatment spent an average of six days in hospital, which was significantly lower than the average 22 days spent in hospital by severe patients who only received standard treatment.
Further analysis revealed that patients treated with the stem cells treatment are three times more likely to recover in less than seven days than those treated with standard therapy, and 67 per cent of the patients who received the stem cells treatment owe this recovery to the new treatment, reported state news agency Wam.
The centre has secured intellectual property rights protection, including copyright and know-how, for the treatment. This means the details of the treatment can be made available for others to replicate via a licensing arrangement, once it gets formal UAE Government approval.
To ensure the treatment was successful and safe, the data from the 73 patients had to be matched to an equal number of historical controls, allowing researchers to compare the treatment outcomes.
The study measured the incidence of adverse effects, mortality rate within 28 days, and time to clinical improvement or discharge from hospital. Before treatment, a patient’s immune response profile, acute phase serum markers, and coagulation testing profile were evaluated.
Patients were excluded from the new treatment arm if they had haemoglobin levels below 10, any blood infections, a history of cancer, or received any treatment that was not part of the standard protocol, such as convalescent plasma therapy. Patients below 18 years old were also excluded from the trial.
Researchers are now looking into establishing the optimal dose needed for patients and if the treatment could be effective in treating other respiratory diseases including asthma, chronic obstructive pulmonary disease, and cystic fibrosis.