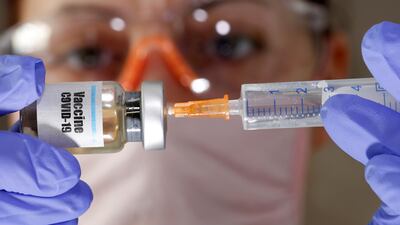
Trials of a coronavirus vaccine are set to take place in the UAE after an agreement was reached with a Chinese medical firm.
Organisers of the programme announced the new partnership at ceremonies in Abu Dhabi, Beijing and Wuhan earlier this week.
If successful, the Phase III trial could result in the vaccine going on to be produced on a large scale within the Emirates.
The initiative is a partnership between China National Biotec Group (CNBG) and Group 42 (G42), a UAE technology company. G42 will carry out the clinical work under the supervision of the Department of Health Abu Dhabi.
Sheikh Abdullah bin Mohammed Al Hamed, Chairman of the Department of Health Abu Dhabi, said the partnership highlighted the UAE’s “broad multifaceted approach to combating the virus”, which also includes research into treatments and enhanced testing.
“The UAE will spare no effort in contributing to solutions to the current pandemic, solutions that will aid humanity’s ability to overcome the current pandemic,” he said.
Abdulrahman Al Owais, the UAE Minister of Health and Prevention, said “now more than ever”, nations needed to promote government and private sector partnerships to make progress.
“It is for this reason that the United Arab Emirates welcomes all contributions by countries of the world, innovative entities and creative individuals who are committed to creating opportunities for joint collaboration towards confronting the threat of Covid-19 and defeating this global pandemic,” he said.
The results of Phase I and Phase II trials of the vaccine in China showed patients had developed antibodies to the virus with no serious adverse reactions.
Officials have not announced when the first vaccinations will take place nor the number of patients involved.
G42 is already a key player in UAE efforts to combat the coronavirus, having built what is described as the second-largest testing laboratory in the world in Masdar City in Abu Dhabi.

The facility is capable of processing tens of thousands of tests per day, greatly enhancing the country’s ability to control the spread of the outbreak.
Given in two doses, the new vaccine has been developed by the Wuhan Institute of Biological Products, which is linked to CNBG’s parent firm, Sinopharm.
CNBG is said to have built a production facility in China with an annual capacity of 200 million doses of the vaccine, which could be used widely from late this year.
During the joint ceremonies, held on Tuesday, UAE officials issued approval certificates for the trials to Chinese government officials and senior CNBG figures.
Dr Ali Obaid Al Dhaheri, the UAE ambassador to China, said the partnership reflected the “spirit of cooperation envisioned by our respective leaderships”.
“This agreement comes during extraordinary times, with a significant global challenge to contend with, one of the greatest tests humanity has faced in the modern era,” he said.
“This partnership is a testament to the robust and deepening UAE-China comprehensive strategic partnership. Healthcare is an important and essential aspect of this co-operation.
"This significant project launch offers a fine example of the greater work our nations can achieve together in future.”
The earlier clinical trials of the vaccine began in April and took place in Henan province in central China, involving 1,120 volunteers aged 18 to 59.
China has a total of five coronavirus vaccine programmes in progress, understood to be more than any other country in the world. It is also organising Phase III clinical trials of other vaccines in Canada and Brazil.
Globally, more than 90 vaccines have been developed, although the scientific journal Nature has said that just "a handful" are likely to end up being used in large-scale vaccination programmes.
What is a Phase III trial?
Phase III clinical trials typically take place after a drug or vaccine has been shown to be effective on a small number of people.
The trial increases participants up to around 3,000, some of whom receive the medicine while others, part of a control group, do not.
To prevent bias, patients are assigned to each group randomly and are not told which category they fall into.
The reason is to prevent any possibility of their perception of their condition being affected.
Many researchers involved in a trial may also not be told who is in which group, again designed to stop their interpretation of the results being influenced.
The scenario is known as a double-blind.
Often in phase III trials, a new medicine is being compared against an existing one.
In the case of a coronavirus vaccine being developed by the University of Oxford in the UK, for example, some volunteers receive the coronavirus vaccine, while control group members are given a meningitis vaccine.
Phase III trials often take place in multiple countries to ensure that a drug or vaccine is trialled on people who are genetically diverse.
Highlighting this, Dr Jenniffer Mabuka-Maroa, programme manager for the African Academy of Sciences, said in April that responses to drugs or vaccines “are complicated and can be influenced by, among other things, human genetics”.
“Different people will respond differently to different drugs and vaccines,” she said.
With the coronavirus, it is also important for vaccine trials to happen in countries where there is a likelihood that people will be exposed to the pathogen.
This is because researchers are assessing, in part, if those given the medicine can go on to develop Covid-19. Any trial would be ineffective if conducted in a location where participants had zero risk of exposure to the virus.
In the case of the Oxford virus programme, clinical trials are taking place in the US, Brazil and the UK.
The US and Brazil have the two highest coronavirus case numbers in the world, making them especially valuable places to hold trials. Another Chinese coronavirus vaccine is also being trialled in Brazil.
Because of the urgent need for a vaccine, timetables for clinical trials are being compressed. Phase III clinical trials can sometimes take several years, but for coronavirus vaccines, this is being reduced to several months.
This means that the coronavirus vaccine being trialled in the UAE could potentially be used in large-scale inoculations by late 2020.
Phase III trials are the last stage before a drug or vaccine is licensed. Phase IV clinical trials typically determine how long immunity lasts or if there are longer-term side effects.